Location: Home >> Detail
TOTAL VIEWS
Immunometabolism. 2021;3(1):e210002. https://doi.org/10.20900/immunometab20210002
1 Biology of Aging Program and Immunomonitoring Platform, Singapore Immunology Network (SIgN), Agency for Science Technology and Research (A*STAR), Immunos Building, Biopolis, Singapore 138648, Singapore
2 Department of Geriatrics, Faculty of Medicine, University of Sherbrooke, Sherbrooke, QC J1K 2R1, Canada
3 Department of Microbiology, National University of Singapore, Singapore 117597, Singapore
* Correspondence: Anis Larbi, Tel.: +65-64070412.
This article belongs to the Virtual Special Issue "Immunometabolism and Aging"
A 20% global increase in the number of obese individuals is likely to occur by 2030. Projections for the US alone suggest that 85% of the population may be overweight or obese by 2030. This is a worrying trend, as obese individuals exhibit many symptoms of metabolic syndrome (MS). In the first section of this review, we cover recent literature describing how obesity and aging have a similar impact on the immune system by contributing to chronic low-grade inflammation. In the second section, we describe potential interventions that could mitigate physiological changes associated with obesity and aging, and discuss future studies that would be necessary to elucidate the impact of obesity on immunity and metabolic health in order to further the advancement of precision medicine.
BMI: Body Mass Index
CDC: Centre for Disease Control and Prevention
CMV: Cytomegalovirus
CRP: C-Reactive Protein
COVID-19: Coronavirus Disease 2019
DNA: Deoxyribonucleic Acid
FFA: Free Fatty Acids
HLADR: Human Leukocyte Antigen DR
HIF: Hypoxia Induce Factor
HPB: Health Promotion Board
IL: Interleukin
mTOR: Mammalian Target of Rapamycin
MS: Metabolic Syndrome
ROS: Reactive Oxidative Species
RNA: Ribonucleic Acid
SASP: Senescent Associated Secretory Phenotype
T2DM: Type 2 Diabetes Mellitus
TAME: Targeting Aging with Metformin
TH: T Helper
TLR4: Toll-like Receptor 4
TNF-α: Tumor Necrosis Factor α
VAT: Visceral Adipose Tissue
US: United States
WHO: World Health Organization
Globally, the population of obese individuals is likely to grow by another 20% before 2030 [1]. In the US alone, 85% of the adult population could be overweight or obese by 2030 [2]. Obese individuals present with high rates of clinical morbidities, such as those described within metabolic syndrome (MS). MS is a cluster of conditions in which individuals present with impaired regulation of various metabolites, such as glucose and lipids [3,4]. This results in a host of symptoms such as hyperglycemia, hyperlipidemia, hypertension and excessive visceral fats. These symptoms are strongly associated with an increased risk of cardiovascular disease, stroke and type 2 diabetes [5]. The rise of obesity in recent decades has been exacerbated by the shift from manual labour to sedentary jobs, as well as changes in eating behaviour where individuals favour a high glucose, high salt and high fat diet [6]. To mitigate the rising healthcare burden caused by obesity, global and local organisations such as the WHO, CDC (US) and HPB (Singapore) have been strongly advocating that people should adopt an active lifestyle and moderate their consumption of unhealthy food [7–9].
In parallel with rising obesity, global populations are also rapidly aging, with the number of elderly (≥60 years of age) expected to reach 1.4 billion in 2030 and 2.1 billion in 2050 [10]. While chronological aging is a natural phenomenon, it is associated with an increased susceptibility to many life-threatening conditions such as infectious disease, cancer, cardiovascular disease and stroke [11,12]. Thus putting a strain on global healthcare systems. The concomitant rise in obese and aging populations is set to present global healthcare systems with unprecedented challenges.
While obesity has been traditionally determined by body mass index (BMI), this metric alone may be insufficient to identify individuals at high risk of developing obesity-related morbidities due to their disproportionate fat and nutrient density. There is thus a clinical need to broaden our definition of obesity to incorporate parameters such as metabolism and visceral fat distribution, especially in the case of elderly individuals, who often have irregular fat distribution that is masked by acceptable BMI criteria [13]. With these perspectives, we hope to stimulate further research exploring the interactions between obesity, aging and immunity. In particular, insights into the immune aspects of ageing and obesity may pave the way for potential interventions that could alleviate the healthcare burden.
Low-Grade Chronic inflammation is a phenomenon central to both obesity and aging. Obese individuals have been shown to exhibit higher systemic levels of pro-inflammatory cytokines such as CRP, TNFα and IL-6 compared to healthy individuals. A similar phenomenon that occurs during aging has been described by Claudio Franceschi as ‘inflammaging’ [14–17]. While regulated acute inflammation is a necessary immune response to resolve infections and encourage tissue expansion, chronic inflammation is detrimental to the host [18]. Studies have shown that obesity-associated inflammation in adipose tissue damages the liver by encouraging the release of reactive oxygen species (ROS) and promoting cell death, leading to hepatocarcinogenesis. This is due to the secretion of excessive free fatty acids (FFAs) by hypertrophic adipocytes, which promotes the local release of pro-inflammatory cytokines [19–27]. Apart from damaging the liver, obesity-associated inflammation can also cause β-cell dysfunction and impaired glucose metabolism [28], which can lead to Type 2 Diabetes Mellitus (T2DM). In particular, the pro-inflammatory cytokine TNFα has been shown to promote glucose intolerance [29] and impair glucose metabolism, resulting in high glucose levels that contribute to endothelial inflammation [30].
In addition to exhibiting dysregulated metabolism and inflammation, obese individuals on the higher end of the BMI spectrum (>35) have also been shown to benefit less from influenza vaccination [31,32]. While the mechanisms remain unclear, this could be due to the accumulation of visceral adipose tissue (VAT). This generates a chronic pro-inflammatory milieu as adipocytes are activated, begin expressing HLADR, and activate adipose resident T cells through the Stat3 pathway [33,34]. In addition, accumulated VAT has been shown to have substantial infiltration of pro-inflammatory immune cells such as macrophages, neutrophils, B cells, TH1 CD4 T cells, TH17 CD4 T cells, γδ T cells and CD8 T cells. Moreover, as the VAT environment is hypoxic and rich in FFA, it promotes the activation of VAT-infiltrating macrophages via the HIF-1α and TLR4 signalling pathways, leading to the production of TNFα and further establishing a pro-inflammatory environment [35–38]. These multiple factors could work in concert to overwhelm the anti-inflammatory cytokines and environment produced by TH2 CD4 T cells, iNKT and Treg cells [39–45].
Senescent cells (i.e., fibroblasts, T cells, B cells and NK cells) that exhibit a senescent secretory associated phenotype (SASP) are often implicated in the sustenance of chronic low-grade inflammation in aging, because these cells are able to secrete pro-inflammatory cytokines without antigenic stimulation [46–51]. The accumulation of these senescent cells could be due to a dysfunctional immune system; indeed, Ovadya et al. have shown that an impaired immune system accelerates the accumulation of senescent cells [52]. Two main factors have been implicated in the accumulation of senescent T cells with age: an individual’s cumulative infection history over the course of their lifetime (exacerbated by chronic infections), and thymic involution [51,53–55]. In support of the former, studies showed that age-matched individuals (from age 1 to >60) with CMV infection exhibited higher proportions of senescent, exhausted and terminally differentiated T-cells [56,57].
Based on the similarities in the detrimental health impacts of obesity and aging, the term ‘adipaging’ has been used to describe the elevated levels of inflammation associated with chronic obesity, as obese individuals tend to be characterised by higher biological age [58]. Due to the low-grade chronic inflammation generated in the VAT, obesity could possibly induce telomere attrition and higher oxidative stress, and have negative influences on mitochondria and genomic stability [59–62]. These phenomena are reminiscent of the various hallmarks of aging [63]. However, as obesity and aging are not mutually exclusive, there is potential for obesity to compound the impact of aging on various physiological systems. This has been observed in a condition described as sarcopenic obesity, where muscle loss is accompanied by fat tissue gain implying a loss of endothelial cell tissue and a concomitant accumulation of fat in the thymus and other organ systems [55,64–66]. An important question to address is how interactions between muscle loss and fat gain affect immunological homeostasis in organs. Many studies have indicated a negative effect that correlates with increasing age, as obesity raises the health-risk in middle-aged adults, but at a rate that declines with increasing age between 60–80 years of age [67]. Nevertheless, later studies have shown that a shift in body fat distribution and an increase in visceral fats with age contribute to a greater likelihood of heart disease and type 2 diabetes [68].
The complex interactions between aging and obesity suggest that determining health risk profiles by BMI alone may be restrictive, especially for the elderly as visceral fats contribute less towards BMI. While most of the focus has been on adults and the elderly, the impact of obesity has not been thoroughly investigated in early life (i.e., new-born to the 2nd decade of life). Notably, maternal gestational diabetes has been observed to contribute to obesity in early life [69–72]. As children in the modern era navigate towards less physically demanding lifestyles [73], they may be more prone to accelerated aging caused by obesity, and the early onset of immunological aging may have grave implications on their immunological health and well-being in later life. In light of this, future studies should focus on this younger demographic. While the mechanisms for immune dysregulation and the source of pro-inflammatory cytokines are different in obesity and aging, both affect the functional capacity of the immune system and increase basal systemic inflammation levels. As such, both conditions predispose individuals to an increased susceptibility to infectious diseases, as seen in the current COVID-19 pandemic [74–77].
Caloric restriction and intermittent fasting have been suggested as potential dietary interventions to counteract the immune effects of aging and obesity. Both have been shown to reduce oxidative stress, improve mitochondrial function and result in BMI reduction due to limited caloric intake [78,79]. Besides dietary intervention, drug interventions involving Rapamycin and Metformin have also been proposed and tested. Rapamycin, which targets the mTOR pathway, has been shown to improve vaccine efficacy in mice [80]. RAD001, an mTOR inhibitor, was shown to increase immune function and boost influenza vaccine responses in elderly individuals [81,82]. The latter is an important discovery, as vaccination remains one of the most cost-effective healthcare strategies for controlling infections, but has been shown to have reduced efficacy in elderly individuals. While Metformin is widely used to improve insulin sensitivity in diabetic patients, recent studies have shown that metformin could be a tool to ameliorate aging. The TAME trial attempts to capitalise on this discovery by repurposing the drug for the mitigation of inflammaging [83].
Since senescent cells contribute to low-grade chronic inflammation in the host by releasing inflammatory mediators, there is a growing focus on the use of senolytics to reduce inflammaging by promoting the removal of senescent cells [84,85]. Senolytics work by inhibiting anti-apoptotic signalling such as via the PI3K/AKT-, p53/p21/serpine-, HIF-1α- and BCL-2/BCL-XL pathways. A recent study demonstrated that the combinatorial use of dasatinib and quercetin can promote pre-adipocyte differentiation, reduce macrophage infiltration and improve glucose homeostasis and insulin sensitivity by eliminating senescent cells from adipose tissue [86]. Besides dasatinib and quercetin, navitoclax and fistein are also currently being tested for senescent cell removal [87,88]. As each drug has a narrow range of specificity for certain senescent cell types, as well as different side effects, it is important to explore a wide range of senolytics to optimise their usage. For example, we now understand that dasatinib selectively targets senescent adipose progenitors and quercetin eliminates senescent endothelial cells [84]. The specificity of senolytics is likely to limit their toxicity, while the capacity of some drugs to specifically target adipocyte progeny suggests that they may be even more advantageous in the context of obesity [89,90]. Collectively, senolytics aim to improve quality of life by negating dysregulated metabolic processes and inflammation by targeting cellular sources of immune activation.
Obesity is a chronic non-communicable disease that is associated with cardiovascular disease and diabetes mellitus [91]. Research in the past decade has shown that obesity can manifest itself in different ways. BMI has been adopted as a traditional approach that classifies individuals into categories such as underweight, normal, overweight and class 1, 2 and 3 obesity based on height and weight [92]. While this is efficient and cost-effective, it requires further calibration to determine guidelines that are suitable for different ethnicities [92]. To circumvent ethnic-specific differences in build, studies have included ethnic-based BMI and the measurement of visceral fat mass to more sensitively stratify individuals according to their predisposition towards cardiovascular diseases and diabetes [93,94]. However, this approach is still not sensitive enough to identify people that have metabolic obesity (thin-fat) but exhibit normal weight [95,96]. Similarly, people who are obese in terms of BMI but exhibit normal metabolic physiology (fat-thin) may be inaccurately classified by this system [97,98]. However, there are many overlaps in the health risks faced by thin-fat, fat-thin and obese individuals. Whether these thin-fat and fat-thin phenotypes exist along the same obesity-associated morbidity risk spectrum that includes obese individuals is unknown and requires further study. For individuals with these different types of irregular fat distribution, it is pertinent to investigate how their unique physiology impacts their metabolism and immune system, so that we have a more universal and accurate method to profile at-risk individuals for therapeutic interventions.
Lifestyle modifications such as diet and physical activity, and drug interventions such as metformin, are potential cost-effective interventions for aging and obesity. However, the mechanisms by which they operate are not well understood. The field of epigenetics, which studies the impact of histone modifications on the repression or activation of genes, is a useful tool that can demonstrate how lifestyle and the environment can affect cellular behaviour. Due to differences in cellular environment, interventions can have a diverse range of effects on their targets. Single-cell epigenetics may provide better resolution to help us make sense of this heterogeneity. In addition, non-coding RNA such as microRNA could play an important role in the regulation of pro-inflammatory genes. The study of metabolism through the lens of mitochondrial behaviour is also an important tool to understand how obesity and aging give rise to abnormal metabolite and ROS distribution. Collectively, future studies in these areas should yield clearer insights that may help clinicians negotiate the diverse individual responses to intervention.
In conclusion, the field of immunometabolism is gaining traction, but several gaps in our knowledge exist that require further study, especially in light of the rising trends of aging and obesity. With advanced technologies such as single-cell epigenetics, RNA-seq, metabolomics, and flow cytometry, researchers are equipped with the tools to identify the mechanisms underlying these processes at both the cellular and molecular level. The latter is critical for identifying specific risks for personalised medicine (Figure 1).
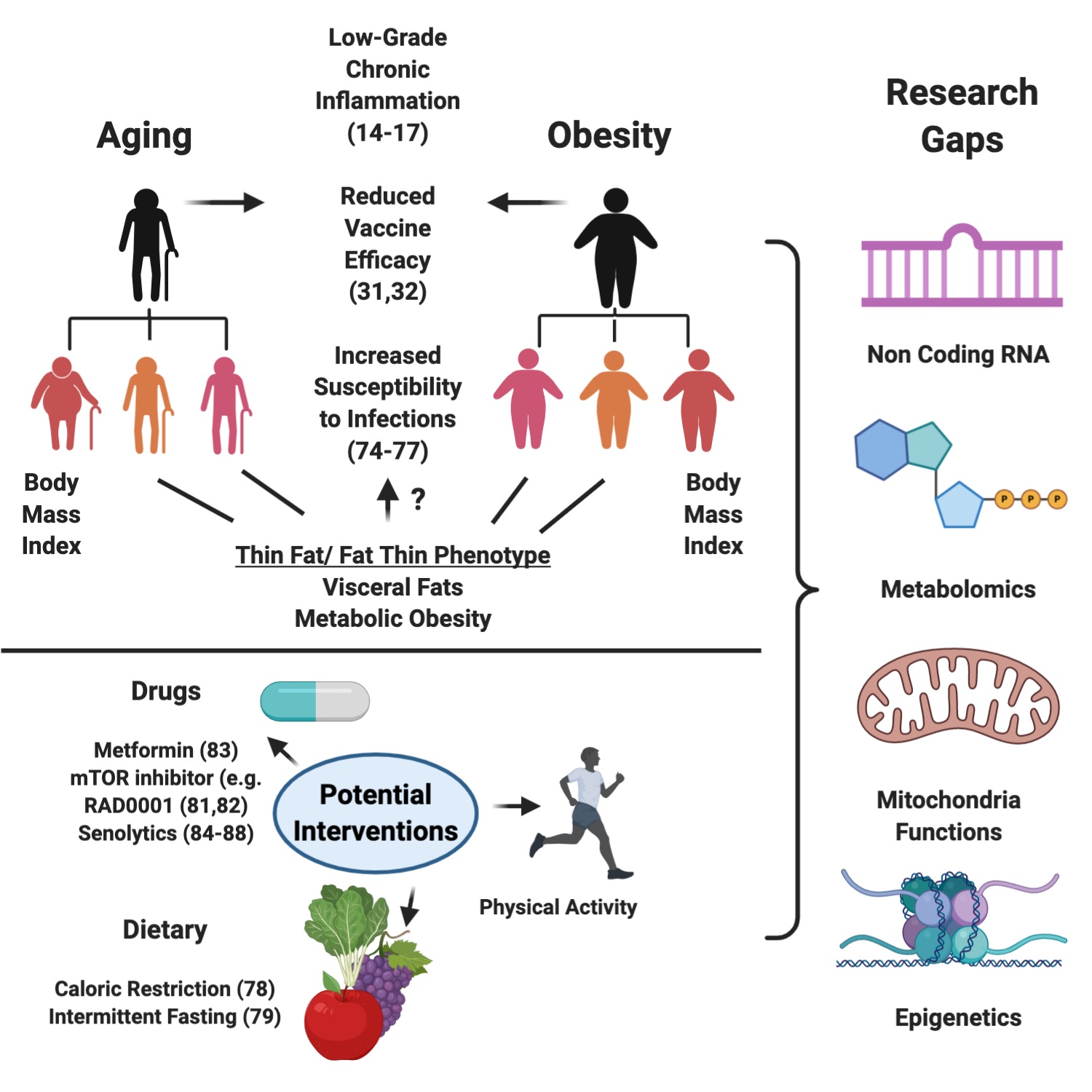 Figure 1. Illustration of the detrimental consequences of Aging and Obesity, the different facets of obesity, potential interventions, and research gaps in the field. Created with Biorender.com.
Figure 1. Illustration of the detrimental consequences of Aging and Obesity, the different facets of obesity, potential interventions, and research gaps in the field. Created with Biorender.com.
WX and AL wrote the manuscript.
We will like to thank Dr Wong Chun Lin, Glenn,Dr Youyi Hwang and Dr Emma Kay Richardson for proofreading and improving the quality of the manuscript.
The authors declare no conflict of interest.
This research was funded by the Singapore Immunology Network and the Agency for Science Technology and Research (a*star).
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
87.
88.
89.
90.
91.
92.
93.
94.
95.
96.
97.
98.
Xu W, Larbi A. Gearing up for the Future: Mitigating Dysregulated Inflammation in Aging and Facets of Obesity. Immunometabolism. 2020;3(1):e210002. https://doi.org/10.20900/immunometab20210002

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions