Location: Home >> Detail
TOTAL VIEWS
Immunometabolism. 2020;2(1):e200001. https://doi.org/10.20900/immunometab20200001
1 Laboratory of Hepatology, CHROMETA Department, KU Leuven, Leuven 3000, Belgium
2 Department of Gastroenterology and Hepatology, UZ Leuven, Leuven 3000, Belgium
* Correspondence: Hannelie Korf, Schalk van der Merwe.
This article belongs to the Virtual Special Issue "Immunometabolism and Inflammation"
Obesity is a widespread health condition, which can lead to the development of metabolic disorders, such as type 2 diabetes mellitus, nonalcoholic fatty liver disease and cardiovascular diseases. Obesity is marked by the excessive deposition of fat in adipose tissue sites combined with chronic low-grade inflammation. Within this clinical setting, it is well established that adipose tissue macrophages exhibit prominent roles in regulating inflammation and metabolism. However, aside from these well-established roles, the involvement of microenvironmental cues as well as underlying cellular metabolism in driving immunological fate decisions within macrophages are poorly understood. Here we aim to map the different adipose tissue-derived macrophage subsets, together with their metabolic and functional profiles. Finally, we discuss their potential contribution during homeostasis and disease progression associated with obesity.
Obesity is a global health pandemic that predisposes individuals to develop metabolic disorders, such as type 2 diabetes mellitus and non-alcoholic fatty liver disease (NAFLD) [1]. In order to face this escalating disease burden, there is an urgent need to unravel the molecular mechanisms that prevent the life-threatening metabolic comorbidities in this expanding patient population and to define novel targets for early therapeutic intervention. Although the disease mechanisms remain incompletely understood, it is well established that early inflammatory events within the expanding adipose tissue compartment significantly contribute to dysregulation of metabolic homeostasis [2]. For example, adipose tissue can secrete a variety of adipokines that influence inflammation and insulin resistance. Macrophages and their pro-inflammatory cytokine repertoire are prominent contributors to the adipose tissue secretome and have been identified as key early driver of insulin resistance [3–6]. Furthermore, adipose tissue macrophages can directly influence recruitment of immune cells from the circulation into adipose tissue sites but also in distal organs such as the liver [7–10], and their frequencies correlate with hepatic histopathological severity [11]. Consequently, disruption of macrophages, or their functions, improves insulin sensitivity and abrogated hepatic inflammation and steatosis [12–14]. These findings support a role for macrophages and adipose tissue inflammation in metabolic disorders such as obesity and its complications.
Macrophages are highly diverse cells in terms of functionality, although they all share a common core program directed by lineage-specific transcription factors [15]. Macrophages can be either embryonically seeded in organs where they are maintained through self-renewal [16], or derived from infiltrating bone marrow monocyte precursors [17,18]. Regardless of their origin, they adapt to microenvironmental cues within the niche they reside in and become imprinted with a unique transcriptional signature [19,20]. Technologies such as single-cell RNA sequencing accelerated discoveries in the field and even implicated a variety of niche-specific macrophages co-existing within one organ [21]. Importantly, these macrophage subsets are specialized in exerting functions such as phagocytosis of apoptotic/necrotic cells, secretion of cytokines and growth factors and remodeling of the extracellular matrix, and all of these processes require mobilization of specific intrinsic metabolic processes [22]. Metabolic repurposing could therefore potentially aid in fine-tuning and correcting macrophage malfunctions during a diseased condition. Notably, as proof of concept, our group recently demonstrated that metabolic rewiring improved monocyte functionality in patients with acute-on-chronic liver failure [23]. Nevertheless, it remains poorly understood how these microenvironmental cues affect immune-metabolic functions of macrophages, especially within the lipid overload setting of obesity and its complications. Here we provide an overview of different subtissular niche macrophages with a focus on their immune-metabolic profile within the adipose depot and discuss their potential contribution during disease progression during obesity.
More than a decade ago, some studies suggested that healthy adipose tissue contains alternatively activated macrophages (M2-like). M2-like macrophages exhibit an anti-inflammatory function through the actions of Interleukin-10 (IL-10) and signal transducer and activator of transcription 3 (STAT3) [24]. Additionally, they maintain insulin sensitivity through peroxisome proliferator-activated receptor gamma (PPARγ), which promotes tissue remodeling and consequently resolves inflammation [25,26]. In support of this concept, interfering with M2-like activation by inhibiting expression of parameters within the downstream IL-4 receptor signaling pathway hampered insulin sensitivity via components such as PPARγ, PPARδ and KLF4 [25,27,28]. To support these homeostatic and restorative functions, M2-like macrophages exhibit a metabolic profile that relies on fatty acid oxidation (FAO) to fuel tricarboxylic acid (TCA) cycle-coupled oxidative phosphorylation [29], which relies on free intracellular coenzyme A (CoA) availability as a regulator of oxidation, as recently been described [30,31]. Furthermore, a role for the mammalian target of rapamycin complex 2 (mTORC2) and interferon regulatory factor 4 (IRF4) in M2 polarization have been demonstrated. Towards this end, elevated expression of mTORC2 and IRF4 increased glucose-dependent oxidative phosphorylation and upregulated the characteristic M2 parameters arginase 1 and resistin-like molecule α [32].
In contrast, classically activated (M1-like) macrophages are recruited and retained in adipose tissue in NAFLD subjects, where they secrete pro-inflammatory mediators and disturb insulin sensitivity [6,33,34]. Deletion of the pro-inflammatory signaling molecule IKKβ in myeloid cells preserved insulin sensitivity and reduced adipose tissue inflammation [13]. Similarly, macrophage-specific deletion of stress-activated c-Jun NH2 terminal kinases, JNK, protects against high-fat diet-induced obesity and insulin resistance and reverted M1 polarization [35]. To meet the energy demand required to exert these acute inflammatory actions, M1 macrophages rely on increased glucose uptake and glycolytic flux [29]. They also feature an interrupted TCA cycle whereby citrate and succinate intermediates accumulate within the cell [36]. Interestingly, the build-up of succinate leads to stabilization of hypoxia-inducible factor (HIF)-1α, a master transcriptional regulator of pro-inflammatory and glycolytic genes [37]. HIF-1α regulates important downstream target genes such as the glucose transporters, GLUT1 and GLUT3 [38], as well as the pyruvate dehydrogenase kinase 1 (PDK1), a metabolic checkpoint directing glucose metabolism towards glycolysis and away from oxidative phosphorylation [39,40]. Finally, macrophage HIF-1α was increased in human and mouse Nonalcoholic steatohepatitis subjects and deletion of myeloid HIF-1α impaired macrophage pro-inflammatory function and adipose tissue inflammation while restoring glucose tolerance [41–43].
Notably, in the in vivo setting and especially in obesity, many different stressors can lead to the activation of inflammatory pathways within adipose tissue macrophages [44]. In addition, macrophages can reside in very different subtissular microenvironments where they give rise to a spectrum of subtypes not accounted for in the oversimplified M1/M2 paradigm. Thereby, macrophages can be located in hypoxic regions or can be found in the vicinity of blood vessels, -nerve fibers, -extracellular matrix or -dying adipocytes. To this end, several studies have demonstrated that macrophages exhibit distinct transcriptional signatures and epigenetic traits that are specific to their location [45–47]. This underscores the role of tissue factors in imprinting the macrophage transcriptional program and consequently underlines the importance of studying these tissue microenvironments during obesity and its complications.
As mentioned above, we are only starting to grasp the extent of these macrophage phenotypes and their accompanying metabolic signatures. These various macrophage phenotypes located in the adipose tissue are depicted in Figure 1 and further reviewed here. For instance, it is possible that lipids released by dying adipocytes are excessively engulfed by resident adipose tissue macrophages giving rise to unique macrophage phenotypes. Indeed, recent studies have characterized metabolic signatures of murine adipose tissue, defining two new phenotypes, termed redox-regulatory (Mox) and metabolically activated (MMe) macrophages. Mox macrophages feature enhanced antioxidant gene expression profiles and a quiescent metabolic state, and were reported to be present primarily in lean adipose tissue following truncated oxidized lipid exposure [48]. On the contrary, obese adipose tissue macrophages featured a metabolically activated state (MMe) with increased lipid catabolism and lysosomal biogenesis [49]. Genes such as perilipin-2 and lysosome-associated membrane protein 2 were specifically upregulated in these macrophages. Notably, a cocktail of glucose, insulin and palmitate could recapitulate this metabolically activated state in vitro [50]. Furthermore, MMe macrophages featured a mixed metabolic program with simultaneous upregulation of glycolysis and oxidative phosphorylation pathways following saturated fatty acid exposure. By promoting inflammatory cytokine production as well as lysosomal exocytosis to adipocytes they also participated in both detrimental and beneficial actions during obesity [51]. Additional studies merging metabolomics, transcriptomics and subtissular locations will be critical in further unravelling the potential heterogeneity within MMe macrophages.
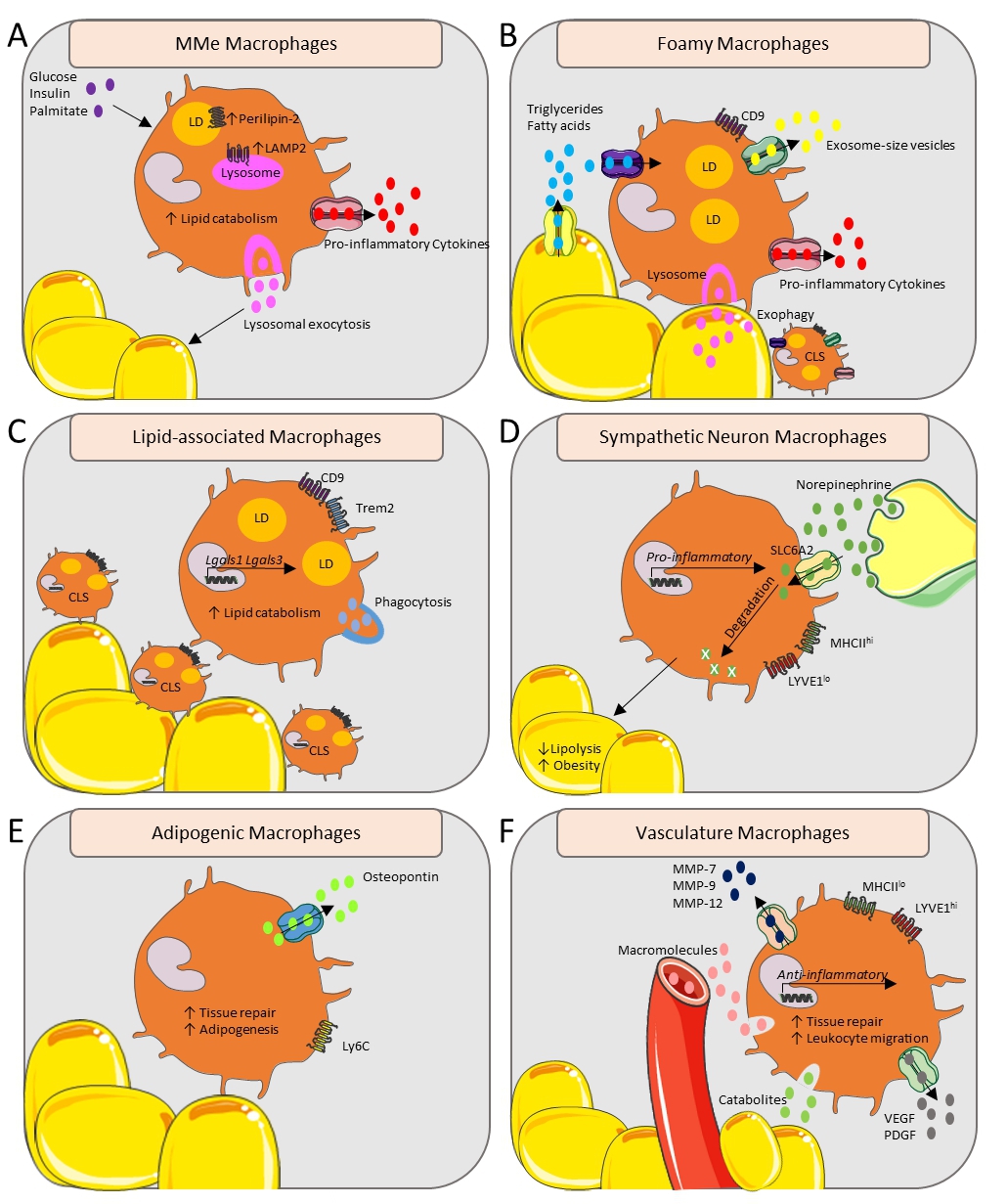 Figure 1. Functional and metabolic overview of adipose tissue macrophages in their subtissular niches. (A) Glucose, insulin and palmitate can induce the metabolically activated (MMe) macrophage state with upregulated Perilipin-2 and LAMP2, leading to increased pro-inflammatory cytokine secretion and inducing lysosomal exocytosis. (B) CD9+ Foamy macrophages form a CLS around the adipocytes, and take up triglycerides and fatty acids released by stressed hypertrophic adipocytes resulting in secretion of exosome-size vesicles and pro-inflammatory cytokines. Macrophages release lysosomal content by forming hydrolytic synapses with stressed adipocytes, called exophagy. (C) The CD9+ lipid-associated macrophages form a CLS and transcribe Lgals1 and Lgals3. The Trem2 lipid sensor drives phagocytosis and lipid catabolism. (D) Norepinephrine, released by sympathetic neurons, is transported via SLC6A2 and is degraded by the sympathetic neuron macrophages. This leads to an upregulated transcription of pro-inflammatory genes and increased obesity. (E) Adipogenic macrophages over-express osteopontin and are involved in tissue repair and adipogenesis. (F) The Lyve1hi vasculature-associated macrophages are localized with the vasculature, engulfing blood-born macromolecules and noxious catabolites, while secreting MMP-7, MMP-9, MMP-12, VEGF and PDGF. Their anti-inflammatory gene transcription profile ensures an involvement in tissue repair and leukocyte migration. CLS, Crown-like structure; LAMP2, Lysosome-associated membrane protein 2; LD, Lipid droplet; LYVE1, Lymphatic vessel endothelial receptor 1; MMe, metabolically activated; MMP-(7, 9, 12), Matrix-metalloproteinase-(7, 9, 12); PDGF, Platelet-derived growth factor; SLC6A2, Sodium-dependent noradrenaline transporter; VEGF, Vascular endothelial growth factor.
Figure 1. Functional and metabolic overview of adipose tissue macrophages in their subtissular niches. (A) Glucose, insulin and palmitate can induce the metabolically activated (MMe) macrophage state with upregulated Perilipin-2 and LAMP2, leading to increased pro-inflammatory cytokine secretion and inducing lysosomal exocytosis. (B) CD9+ Foamy macrophages form a CLS around the adipocytes, and take up triglycerides and fatty acids released by stressed hypertrophic adipocytes resulting in secretion of exosome-size vesicles and pro-inflammatory cytokines. Macrophages release lysosomal content by forming hydrolytic synapses with stressed adipocytes, called exophagy. (C) The CD9+ lipid-associated macrophages form a CLS and transcribe Lgals1 and Lgals3. The Trem2 lipid sensor drives phagocytosis and lipid catabolism. (D) Norepinephrine, released by sympathetic neurons, is transported via SLC6A2 and is degraded by the sympathetic neuron macrophages. This leads to an upregulated transcription of pro-inflammatory genes and increased obesity. (E) Adipogenic macrophages over-express osteopontin and are involved in tissue repair and adipogenesis. (F) The Lyve1hi vasculature-associated macrophages are localized with the vasculature, engulfing blood-born macromolecules and noxious catabolites, while secreting MMP-7, MMP-9, MMP-12, VEGF and PDGF. Their anti-inflammatory gene transcription profile ensures an involvement in tissue repair and leukocyte migration. CLS, Crown-like structure; LAMP2, Lysosome-associated membrane protein 2; LD, Lipid droplet; LYVE1, Lymphatic vessel endothelial receptor 1; MMe, metabolically activated; MMP-(7, 9, 12), Matrix-metalloproteinase-(7, 9, 12); PDGF, Platelet-derived growth factor; SLC6A2, Sodium-dependent noradrenaline transporter; VEGF, Vascular endothelial growth factor.
Foamy macrophages—clearing the dead and fueling inflammation and systemic insulin resistance
Under normal conditions, resident adipose tissue macrophages phagocytose adipocyte debris in order to maintain normal adipocyte turnover and tissue homeostasis. However, during obesity progression, adipocytes undergo plasma membrane rupture, endoplasmic reticulum stress and necrosis-like death [33,52]. Following this event, macrophages rapidly surround and engulf the dying adipocytes, thereby creating a characteristic microenvironment known as a crown-like structure (CLS). Because of the size of the stressed hypertrophied adipocytes, macrophages implement an innovative mechanism by forming hydrolytic synapses in which they secrete their lysosomal content to ingest the dying adipocytes—a process termed exophagy [53]. The enlarged insulin-resistant adipocytes also release triglycerides and non-esterified fatty acids that are continuously scavenged by surrounding macrophages. Combined, this heightened demand on their endocytic capacity may overwhelm the underlying metabolic state giving rise to metabolically activated macrophages (as described above) [49–51]. Additionally, this process may impede efficient dead cell clearance, similar to what happens in foam cells within atherosclerotic plaques [54], culminating in an aberrant inflammatory response [55]. Important differences between foamy macrophages described here and those present in atherosclerotic plaques also exist. For example, excessive lipid loading appears to suppress inflammatory responses due to a defective pentose phosphate pathway [56–58].
During obesity however, an important study by Hill et al. describes a CD9+ CLS macrophage population that exhibit a crucial role in storage of excess lipids and that express genes related to lysosomal-dependent lipid metabolism [59]. Interestingly, the authors of this latter study suggest that CD9+ macrophages metabolically resemble activated macrophages (see above) as well as the previously described CD11chigh macrophages [60], since they similarly reside predominantly in the CLS and secrete pro-inflammatory cytokines. Implicating a pathogenic role for these macrophages, the authors demonstrated that adoptive transfer of CD9+ macrophages into healthy animals was sufficient to propagate obesity-associated adipose tissue inflammation [59]. CD9+ macrophages also seem to play a role in regulating metabolism through secretion of exosome-size vesicles. Indeed, miRNA containing extracellular vesicles (e.g., miR-155) constitute an important part of the adipose tissue macrophage secretome and act as a signaling mechanism to regulate local and systemic insulin signaling [61].
Lipid-associated macrophages—regulators of adipocyte hypertrophy
Further refining the diversity of macrophages during obesity, an elegant study by the group of Ido Amit characterized a number of distinct myeloid cell populations within adipose tissue [62]. One population resembled the transcriptional profile of interstitial perivascular macrophages (described below) [63], and another fitted the signature described by Hill et al., described as CD9+ macrophages (described above) [59]. Strikingly, their results implicate the presence of a third unique macrophage subset, which they termed lipid-associated macrophages (LAMs). The authors demonstrate that LAMs are derived from circulating monocytes and arise specifically under obesity conditions where they are positioned around enlarged adipocytes in crown-like structures [62]. Although also expressing CD9, LAMs seem to be distinct from the pro-inflammatory subset described by Hill et al., and express genes associated with immune suppression such as Lgals1 and Lgals3 [59,62]. The transcriptional signature of LAMs is very close to that described for disease-associated microglia (DAM) cells in the brains of subjects with neurodegenerative disorders and in aortic macrophages during atherosclerosis [64,65]. Another important finding was that LAMs played a crucial role in preventing adipocyte hypertrophy through a Trem2-mediated mechanism. Trem2 acted as a lipid sensor, driving a gene expression program involved in phagocytosis, lipid catabolism and energy metabolism [62]. Abrogating Trem2 signaling caused massive adipocyte hypertrophy, systemic hypercholesterolemia, inflammation and glucose intolerance [62]. This study supports a beneficial role for LAMs in obesity and potentially in other disease-associated comorbidities such as NAFLD.
Sympathetic Neuron Macrophages—Soaking up Neuronal InsultsRecently also a subset of sympathetic neuron-associated macrophages (SAMs) within adipose tissue has been discovered [66]. Sympathetic neurons typically produce the neurotransmitter norepinephrine (NE) that facilitates lipolysis and fat mass reduction. Under stress conditions, however, overproduction and systemic NE can lead to hypertension and cardiopathy due to its direct action on cardiovascular tissues [67]. In this regard, Pirzgalska et al., demonstrated that SAMs play a tissue-protective role by scavenging and catabolizing regional NE levels, thereby serving as a local sink that prevents the dangerous effects of systemically increased NE [66]. Such tissue-protective responses have also been described in the intestinal muscularis nerve-associated macrophages that protect against pathogenic insults via B2 adrenergic receptor signaling [68]. In sharp contrast to the anti-inflammatory state of intestinal nerve-associated Cx3cr1-GFP+ macrophages, SAMs exhibit a pro-inflammatory profile at steady state [66,68]. Regardless of this functional difference, the importance of this population in obesity has been highlighted by the fact that ablation of NE importer, SLC6A2, in these macrophages induced weight loss and lipid mobilization [66]. Along the same lines of investigation, a complementary study demonstrated that brown adipose tissue deletion of macrophage methyl-CpG binding protein 2 (MECP2), a factor important in neurodevelopment, resulted in spontaneous obesity [69]. This was attributed to the inhibition of brown adipose tissue sympathetic innervation, and thus a reduction of NE tissue levels, ultimately leading to altered thermogenesis [69]. Finally, such nerve-associated macrophages appear to be present in a number of different tissues, and at least in mouse models they exhibit a common LYVE1loMHCIIhigh phenotype [70]. This common signature include genes such as Axl, Ccr2, Cx3cr1 and MHCII-related genes such as H2-DMa, H2-Aa, H2-Eb1, H2-Ab1, CD74 and H2-K1 [70]. Combined, these studies highlight the fact that macrophages associated with the sympathetic neuronal system exhibit specialized molecular programs and thus they provide insight into neuronal-macrophage crosstalk mechanisms.
Adipogenic Macrophages—Guiding Adipocyte FormationDuring pathological conditions such as NALFD, where chronic over nutrition prevails, not only the size but also the number of adipocytes increases to compensate for the excessive lipid availability. In this regard, macrophages participate in the (patho-)physiological remodeling of adipose tissue by guiding new adipocyte formation (adipogenesis). Supporting this concept, a recent study implicated an adipogenic- and tissue reorganization role for monocyte-derived Ly6C macrophages that are uniformly distributed throughout the adipose interstitium [59]. Additionally, a population of osteopontin over-expressing adipose tissue macrophages has been demonstrated to establish an adipogenic niche for tissue repair and remodeling in diet-induced obesity models [71]. The authors of this study also demonstrated the importance of this population by showing that osteopontin-deficient mice fail to form these adipogenic nodes. Additional studies are required to further characterize this subset and its importance in obesity and its complications.
Vasculature Macrophages—Gatekeepers to Systemic CirculationWith the expansion of the adipose tissue, local macrophages have been implicated to direct the formation of new blood vessels and as such, play a role in the process of angiogenesis. These macrophages express lymphatic vessel endothelial receptor 1 (LYVE1) and secrete tissue remodeling factors (e.g., matrix metalloproteinase (MMP)-7, MMP-9, MMP-12), and factors promoting the formation of endothelial cell tubes (e.g., VEGF, PDGF) [72,73]. A recent study provides a comprehensive overview of the transcriptome, phenotype and tissue-localization regarding these vasculature-associated macrophages (VAMs) [63]. More specifically, the authors show that steady-state VAMs display an anti-inflammatory gene signature, are self-maintaining and exhibit an extremely rapid capacity to engulf blood-born macromolecules or noxious catabolites from the surrounding adipose tissue [63]. Strikingly, VAMs are suggested to be highly sensitive to cell death since they are rapidly depleted under acute infectious- or metabolic stress [63]. The loss of these valuable macrophages seems to be only transient, as the vascular niche can be repopulated through the recruitment of a monocyte-derived pre-VAM population [63]. Finally, another important study characterized VAMs by reporting that they exhibit a LYVE1hiMHCIIlo phenotype, and that this was a conserved characteristic for infiltrating monocyte-derived cells across a number of different tissues, including fat. Notably, the common gene signature that depicted this population involves genes such as Lyve1, Timd4, CD5l, Fcna and Vsig4 [70]. The authors further demonstrate that LYVE1hiMHCIIlo macrophages expressed higher levels of genes involved in blood vessel morphology, leukocyte migration, tissue repair and fibrosis [70]. Arterial LYVE1 macrophages therefore play a role in maintaining normal vasculature structure, an attribute that could be linked to the regulation of collagen production [74]. Importantly, depleting LYVE1hiMHCIIlo during the induction of fibrosis, exacerbated inflammation and the degree of fibrosis in the lung and heart of their experimental mouse model [70]. It will be intriguing for future studies to explore whether similar mechanisms are in place during fibrosis and cirrhosis development in chronic liver diseases such as NAFLD.
Increasing evidence highlights the close association between adipose tissue macrophage function and their importance in advancing metabolic disorders such as obesity and its complications. Accordingly, adipose tissue macrophage-targeting approaches have underscored their importance in the development of liver inflammation and insulin resistance. Strategies that inhibit the recruitment of macrophages to the adipose tissue compartment are currently being investigated. However, in view of the latest findings regarding the vast diversity of adipose tissue-derived macrophage subsets (reviewed here) it becomes clear that these subsets also portray distinct subtissular-associated functions that can be either beneficial or pathological during disease progression. In addition, it remains to be determined whether these subsets represent distinct subsets or represent merely altered functional states of the same cell. It will therefore be crucial for future studies to integrate transcriptional, metabolic and location signatures at a single-cell level in order to clarify these discrepancies and to define highly specific molecular targets. The latter may reveal important mechanisms that could potentially be exploited to counteract the emerging obesity pandemic.
The authors declare no conflicts of interest.
This work was supported by internal funding from the UZ Leuven (KOOR) as well as by the Flanders fund for scientific research (FWO) (G082018N) and research grants from Gilead Sciences. SvdM is a recipient of a clinical mandate from FWO. HK is supported by an international award from Gilead Sciences.
The authors wish to acknowledge the contribution of Matthew Bird for grammatical proofreading the manuscript.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
Korf H, Boesch M, Feio-Azevedo R, Smets L, Vandecasteele R, van der Merwe S. Depicting the Landscape of Adipose Tissue-Specific Macrophages and Their Immunometabolic Signatures during Obesity. Immunometabolism. 2020;2(1):e200001. https://doi.org/10.20900/immunometab20200001

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions