Location: Home >> Detail
TOTAL VIEWS
Immunometabolism. 2020;2(1):e200008. https://doi.org/10.20900/immunometab20200008
1 Department of Physiology and Biophysics, Institute of Biomedical Sciences, University of Sao Paulo, Sao Paulo 05508-000, Brazil
2 Universidade Tuiuti do Parana, Curitiba, Parana 82010-330, Brazil
3 Laboratory of Physiology and Cell Signaling, Department of Clinical Analyses, Federal University of Paraná, Curitiba, Paraná 80210-170, Brazil
4 Department of Medical Clinic, Faculty of Medicine, University of São Paulo, São Paulo 01246-903, Brazil
5 School of Biomedical Sciences, CHIRI Biosciences, Curtin University, Perth, Western Australia 6845, Australia
6 Interdisciplinary Post-graduate Program in Health Sciences, Cruzeiro of Sul University, Sao Paulo, Sao Paulo 01506-000, Brazil
* Correspondence: Amanda Rabello Crisma.
Leukocytes are potent regulators of adipose tissue biology and whole-body metabolic homeostasis. In lean, non-obese conditions (insulin-sensitive), adipose tissue has innate and adaptive immune cells, including eosinophils, regulatory T cells, invariant NK cells, and M2 macrophages. A vast expansion in adipose tissue occurs in obesity, and this is associated with a marked alteration in the tissue leukocyte profile. There is a marked increase in B cells, CD8+ T cells, NK cells, neutrophils, and M1 macrophages. This condition induces a state of low-grade, chronic inflammation in the adipose tissue, which disrupts whole-body metabolism. Macrophages were the first leukocyte to be discovered in adipose tissue. Due to their proximity to nearby adipocytes, the macrophages are exposed to high levels of fatty acids and other lipids reported in obesity. Lipid uptake by tissue-resident macrophages is essential for their biological actions. Specifically, lipid uptake and metabolism, particularly of long-chain saturated fatty acids, activate inflammatory signaling pathways, potentiating adipose tissue inflammation, and metabolic dysfunction. Obesity exhibits increased fatty acid levels within the adipose tissue microenvironment. The increased lipid accumulation in the resident macrophages reflects the fatty acid composition of the adipocytes. The dietary fatty acid determines the fatty acid composition of the adipose tissue. Macrophages then accumulate fatty acids indirectly provided by the diet. The composition varies with the acyl chain length, e.g., short-, medium-, or long-chain, and saturated fatty acids. These fatty acids have wide-ranging effects on macrophages. We described herein in detail the impact of the different dietary fatty acids on macrophage functions. Shortly, long-chain saturated fatty acids are pro-inflammatory, whereas medium-chain fatty acids are relatively benign. Long-chain unsaturated fatty acids often antagonize the pro-inflammatory effects of long-chain saturated fatty acids.
Macrophages play an essential role in the orchestration of both innate and acquired immune responses. They are versatile cells that exert a wide range of biological functions that include not only defending the organism against pathogenic agents but also participating in tissue homeostasis (in both steady state and injury) and remodeling [1,2]. Elie Metchnikoff was the first to describe macrophages in the 1880s. He stated that macrophages play a central role in the inflammatory response due to their phagocytic nature, which was called theory of cellular immunity [3]. After Metchnikoff, the great diversity of macrophage-like cells observed in both vertebrates and invertebrates led to the concept of the mononuclear phagocytic system (MPS) model proposed by Van Furth and Cohn [4] in an attempt to unify diverse populations of cells into one group [1,5]. According to this model, all tissue macrophages originate from blood monocytes, despite their biological, developmental, functional, and molecular diversity [4,5]. The first view of MPS suggests that recruited macrophages play a crucial role during inflammatory processes, whereas tissue-resident ones are important to local homeostasis [6]. However, the considerable variation of morphologic features, biological functions, and anatomic location [7,8], as well as the lack of a unique marker to distinguish recruited from tissue macrophages [5], led to a revision of the traditional MPS framework and the expansion of this model by including the concept of polarization [5,9].
The origin of macrophages has been extensively reviewed [5,6,8,10,11]. Differences in ontogeny are not sufficient to explain the diversity of macrophages, and functional classifications based on inflammatory states. The most recognized macrophage states are activated macrophages (M1) and alternatively activated macrophages (M2) [6,8]. The microenvironment conditions drive the polarization of macrophages to one type or another. The different activation programs lead the macrophages to carry out the appropriate functions according to the inflammatory state [6]. M1 macrophages utilize arginine metabolism for NO production. They exhibit high expression of pro-inflammatory cytokines (e.g., tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6)), chemokines (e.g., C-X-C chemokine receptor type 1 (CXCR1) and C-X-C chemokine receptor type 2 (CXCR2)). M1 macrophages also show a high production of reactive oxygen species (ROS). These cells then have enhanced microbicidal, inflammatory, and tumoricidal capacity. M2 macrophages utilize arginine metabolism to drive ornithine and polyamine production. They are associated with high expression of anti-inflammatory cytokines (e.g., interleukin 4 (IL-4), interleukin 10 (IL-10), and transforming growth factor β (TGF-β)), which allows them to promote tissue remodeling and dampen inflammation by exerting immunoregulatory effects [6,12]. However, this binary classification is just a simplified model to describe the functional diversity of macrophages, and many subsets other than M1 and M2 have been identified [6].
Besides macrophage polarization, transcriptomic studies identified considerable diversity in gene expression profiles in mouse macrophages from different sites [13,14]. The Immunological Genome (ImmGen) Project [15] addressed this issue. Peritoneal, red pulp splenic, lung, and brain macrophages (microglia) revealed an enormous diversity among them. Classical antigenic protein markers such as F4/80 (adhesion and phagocytic receptor present in mouse macrophages) [16,17], CD68-(oxidized LDL receptor) [18] and CD115 (receptor for M-CSF) [19], are routinely used to identify macrophages, but problems arise for sub-type discrimination. However, CD14 and CD64 are additional important markers [14], being related to LPS (CD14) and IgG (CD64) binding [20,21].
Different stimuli induce cytokine production by macrophages (e.g., self-danger signal, microbial stimulus, ROS production). The pattern recognition receptors (PRRs) include Toll-like receptors (TLR) [22,23], and some inflammatory receptors, interleukin-18 receptor (IL-18R), and IL-1R, which require the signaling protein myeloid differentiation primary response gene 88 (Myd88), participate in the induction of pro-inflammatory cytokines expression [22,24]. Myd88 is an adapted molecule that recruits IL-1R-associated kinase (IRAK) family [25], which leads to the activation of the mitogen activated protein kinases (MAPK) and nuclear factor κβ (NF-κB) pathways [24,25].
NOD-like receptors (NLRs), recognize self and non-self-antigens, are characterized by a central nucleotide-binding and oligomerization (NACHT) domain, which is the only common region among NLR family members [26,27]. NLR family members, named inflammasomes, play an essential role in innate immunity. These receptors are involved in the activation of caspases [28]. The NACHT, LRR, PYD domains-containing protein 3 (NLRP3) inflammasome is one of these complexes that control the activation of (IL)-1 family cytokines: IL-1b and interleukin 18 (IL-18) [28,29] in macrophages.
We described herein the main immunological and functional modifications in macrophages caused by saturated, ω-3, ω-6, ω-9, and ω-7 fatty acid treatments either in vitro or in vivo.
Saturated, ω-3, ω-6, ω-9, and ω-7 families are the main fatty acids found in the diet. ω-3 and ω-6 fatty acids are essential fatty acids because the organism cannot synthesize them, so they have to be obtained in the diet. Safflower and soybean oil are common sources of ω-6 fatty acids. Marine fish are the primary source of ω-3 fatty acids due to their diet rich in phytoplankton and zooplankton (the real sources of omega-3 fatty acids) [30]. Saturated fatty acids are naturally present in many foods, such as fatty beef, pork, and dairy products. Rich sources of ω-7 fatty acids are macadamia oil and sea buckthorn oil.
The main long-chain SFAs (16 or more carbon atoms in the molecule) in mammals are palmitic (16:0) and stearic (18:0) acids. Animal fat products, as whole milk dairy products and fatty meats, are the primary source of this class of fatty acids. Most studies to investigate the effect of SFA on macrophage function used palmitic and stearic acids. Medium-chain fatty acids were also studied, particularly myristic, caprylic, and lauric acids (Table 1). Lokesh and Wrann (1984) [31] reported that treatment with palmitic acid decreases phagocytosis. However, this fatty acid did not change the capacity of mouse macrophages to kill bacteria. Palmitic acid also did not change the production of superoxide after either stimulation with phorbol myristate acetate (PMA) or opsonized zymosan.
In the same way, the authors [31] described that peritoneal macrophages enriched with myristic or palmitic acids display reduced phagocytic capacity and enhanced adhesion to plastic culture dishes. In contrast, enrichment with polyunsaturated fatty acids increases phagocytosis. In human monocyte-derived macrophages treated with stearic acid, an increase in the effector function and decreased colony-forming units (CFU) of M. tuberculosis was observed [32].
Nitric oxide (NO) is an important effector molecule of cytotoxic activated macrophages [60]. Palmitic and stearic acids increase NO production by J774 macrophages at low doses (up to 10 μM); however, at high concentrations (above 50 μM), these fatty acids lead to the opposite effect, including a decrease of NO production [41]. This finding is not a consensus since increased inducible nitric oxide synthase (iNOS) expression and NO production occurred in J744 [61] and THP-1 [34] cell lines treated with high doses of palmitic acid (above 400 μM).
Treatment of primary and cell line macrophages with palmitic acid has been reported to increase IL-1β [43–45,50,57] TNF-α [43,46,47,62], IL-6 [47], IL-8 [48], IL-18 [35], monocyte chemoattractant protein-1 (MCP-1) [49,50], interferon gamma-inducible factor (IP-10) [51] chemokine (C-C motif) ligand 2 (CCL2) [49]. Concerning IL-10 production, treatment of cell lines with palmitic acid leads to both increase [49] or decrease [39]. Some authors described a dose-dependent effect of palmitic acid: low doses enhance the production of proinflammatory cytokines, whereas high doses (above 200 μM) reduce the production of TNF-α and IL-6 by THP-1 cells [34]. Stearic acid promotes the same enhancing effect on TNF-α [32,39,46,59], IL-6 [32,39,59], IL-8 [32,39] IL-12, macrophage inflammatory protein-1 alpha and beta (MIP-1α and MIP-1β) [32] production in both primary and cell line macrophages. Pararasa et al. (2016) [59] escribed decreased IL-10 production by THP-1 cells after treatment with stearic acid. In line with palmitic and stearic acids, myristic acid also has a stimulatory effect on the production of TNF-α [39,59], IL-6, and IL-8 [39] by macrophages.
In systemic inflammatory conditions (such as obesity), plasma cytokines can originate from adipocytes and macrophages that migrate into the adipose tissue [63–66]. Infiltrating macrophages stimulated by fatty acids released from adipocytes play a crucial role, not only in the establishment of inflammation in this tissue but also in the development of insulin resistance in skeletal muscle and liver, through production and release of inflammatory cytokines [67].
SFAs induce inflammatory responses of macrophages through activation of members of Toll-like receptors (TLR) family TLR2 and TLR4 that play a vital role in the innate immune response [62,63,68,69]. Palmitic acid activates TLR4-dependent signaling pathways, increasing phosphorylation of TAK1 [50], JNK [39], p38 [38,44], and c-Jun [39,48], which leads to activation of the AP-1 transcription factor. Similarly, palmitic acid increases the phosphorylation of nuclear factor of kappa light peptide gene enhancer in B-cells inhibitor α (IκBα), which leads to phosphorylation and translocation of NF-κB p65 to the nucleus [38,50]. Phosphorylation of p38, c-Jun [39], IκBα and NF-κB p65 [39], endoplasmic reticulum stress [40] is also described after treatment of macrophages with stearic acid. Activation of both MAPK signaling and NF-κB pathway by SFAs [52,55,62] leads to the production of cytokines and activation of cyclooxygenase (COX-2) [58] and iNOS. In addition to binding to TLRs, palmitic [54,62] and lauric [53,55] acids enhances TLR4 expression in different macrophage cell lines as well as expression of other receptors involved in the immune response, such as CD36 [37] and CD86 [34]. The effect of SFAs on the expression of surface receptors is dose dependent. Xiu et al. (2016)[34] described the decreased expression of TLR-4, MHC (major histocompatibility complex) class II, CD36, and CD86 after treatment with high doses of palmitic acid (above 200 µM).
Besides the activation of TLRs, palmitic acid also increases cellular ceramide content [70,71]. The sphingolipids play an important cell function as bioactive lipids. Sphingolipids, including ceramide, sphingosine, sphingosine-1-phosphate, and ceramide-1-phosphate, regulate inflammation, cell growth, migration, and angiogenesis [72]. Sphingomyelin hydrolysis generates ceramide. This bioactive lipid mediates the effect of saturated fatty acids on proinflammatory gene expression. TLR4 signaling up-regulates the expression and activity of the enzymes involved in ceramide de novo synthesis [70,71]. LPS treatment increases ceramide synthesis by activating TLR4 signaling. Corroborating these data, inhibition of ceramide content decreased IL-6 production induced by palmitic fatty acid in murine macrophages [70].
In addition to TLRs, the free fatty acid receptors (FFARs) are G protein-coupled receptors (GPCRs) activated by free fatty acids (FFAs). The FFARs play essential roles not only as signaling of fatty acid nutritional components but also as signaling molecules in numerous physiological processes [73]. FFARs vary with the chain length of the FFA that activates each FFAR. Medium- or long-chain FFAs activate FFAR1 and GPR120, GPR84 is activated by medium-chain FFAs, whereas short-chain FFAs activate FFAR2 and FFAR3 [73]. Intestine and pancreatic β-cells highly express FFAR1, which induces the incretin and insulin secretion, respectively [74]. FFAR1 or protein-coupled receptor GPR40 was involved in the increase of IL-6 expression in endothelial cells [75] and on beta-cell apoptosis after palmitic acid stimulation [76].
Saturated fatty acids also activate MAPK signaling and NF-κB pathways [52,53,62], leading to the production of proinflammatory cytokines. As summarized above, saturated fatty acids raise NO and cytokine production by activating TLR-4 and NF-κB signaling. Additionally, these molecules activate the NLRP3 inflammasome [35,77]. Activated NLRP3 triggers the transformation of pro-caspase 1 to caspase 1. This enzyme leads to proteolytic cleavage of pro-IL-1β and pro-IL-18 into their active forms [78,79]. Treatment of primary macrophages with both palmitic and stearic acids increases NLRP3 expression in primary macrophages [35,36,56] in an IRE-1α-dependent way [37]. In THP-1 cells, treatment with stearic acid leads to the same enhancing effect [56,58]. However, palmitic acid at low doses (up to 200 µM) increases NLRP3 expression [56], whereas, at high doses (250 µM), NLRP3 expression does not change [58]. The NLRP3 inflammasome is an oligomer of caspase 1, PYCARD (an adaptor protein), NALP (a NOD-like receptor) and caspase 5 (sometimes) [80,81]. The consequence observed after inflammasome activation is caspase-1, IL-1β, and IL-18 production [81,82].
In summary, SFAs trigger a proinflammatory response in macrophages. TLR4-dependent signaling pathways, as MAPK and NF-κB pathways, activate the NLRP3 inflammasome. The increase in the production of NO, proinflammatory cytokines, and other inflammatory mediators then occur (summarized in Table 1 and Figure 1).
ω-3 PUFAs are beneficial in inflammation conditions associated with disease states, including atherosclerosis, autoimmune disorders, tumor malignancy, and sepsis [83–85]. ω-3 PUFAs may impact many macrophage functions in healthy and diseased states such as phagocytosis, respiratory burst, pathogen killing, and cytokine release (Table 2).
Peritoneal macrophages from rodents fed a commercial laboratory chow contain 15–20% of PUFAs. The main PUFAs are arachidonic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) [33]. Peritoneal macrophages from rats fed purified diets containing linseed oil (rich in ω-3 alpha-linolenic acid) for 6 weeks have increased the percentage of ω-3 PUFAs and decreased of ω-6 PUFAs in their plasma membrane. Reduced synthesis of prostaglandins (PG) [98,99] and leukotriene B4 [123] from arachidonic acid occurs under the latter condition.
Macrophage phagocytic activity is a crucial step in the inflammatory response. Changes in plasma membrane phospholipid composition and fluidity determine the efficiency of macrophages to engulf and kill microorganisms [86]. Both structural organization and fluidity vary with the fatty acid composition of the membrane phospholipids [103]. ω-3 PUFAs increase macrophage phagocytosis activity [33,87,88,101,102], either in vitro or in vivo experiments.
The enrichment of RAW264.7 with ω-3 PUFAs results in a marked increase of phagocytosis activity [101,102]. However, some authors reported the phagocytic capacity of monocytes from healthy human [88,89] and mouse [90] remains unchanged after ω-3 PUFA supplementation. A controversy then continues on ω-3 PUFA effects on macrophage phagocytosis.
Evidence suggests that ω-3 PUFAs impair NF-κB signal transduction [104] and mitogen-activated protein kinase (MAPK) pathway, inhibiting pr-inflammatory cytokine and NO production [105] by macrophages. ω-3 PUFAs inactivate the NF-κB signal transduction pathway through inhibition of IκB phosphorylation [104].
Macrophages treated with ω-3 PUFAs (EPA, DHA, and alpha-linolenic acid) produce significantly less NO due to a decrease in iNOS activity [106–109,124]. On the other hand, in J774 cells, treatment with low concentrations of EPA and DHA increases NO production, whereas at high levels, NO production is decreased [41].
Treatment of lipopolysaccharide (LPS)-stimulated macrophages with EPA, DHA, and alpha-linolenic acid (LNA) decreases the production of TNF-α [110–113,124], IL-1β and IL-6 [109,110,112–116]. EPA also reduces TNF-α and IL-6 expression by macrophages derived from blood mononuclear cells and stimulated with LPS and prostaglandin E2-PGE2 [117].
Decreased cytokine production caused by the addition of ω-3 PUFAs associated with impairment in the NF-κB signaling pathway. Both EPA and DHA down-regulate LPS-induced NF-κB/DNA binding in THP-1 macrophages [115]. However, DHA promotes a more pronounced decrease of macrophage nuclear NF-κB p65 [118] and IL-1β [115–119] expression as compared to EPA. DHA is also more effective than EPA to attenuate LPS-induced pro-inflammatory cytokine production by macrophages [114,115]. Reduction of both nuclear translocation and binding capacity to DNA of p65 subunit, as well as augment of NF-κB inhibitor IκBα by ω-3 PUFAs, impair the production of inflammatory mediators [115].
ω-3 PUFAs facilitate bacterial growth due to their effects on the inhibition of pro-inflammatory cytokines. In fact, in vitro treatment of Mycobacterium tuberculosis-infected murine macrophages with EPA [95] and DHA [120] enhances the units of mycobacteria due to inhibition of TNF-α production.
T helper 1 cytokines (interferon γ (IFN-γ), TNF-α, IL-6) and T helper 2 cells (IL-4 and IL-13) have a crucial role in macrophage polarization and activation [125,126] during homeostasis and inflammatory diseases [127,128]. Since 1989 it was observed that ω-3 supplementation decreased the production of IL-1β and TNF-α of mononuclear cells [92].
Mice with androgen-sensitive prostate cancer fed with a diet containing ω-3 PUFAs during 35 days had decreased expression of M1 (F4/80, iNOS) M2 (ARG1) markers and IL-6, TNF-α, IL-10 tumor-infiltrating macrophages [93]. Some trials have been showing that ω-3 supplementation during six weeks before prostatectomy surgery, decrease Ki-67 (proliferation marker), prostate cancer development, and progression [129,130].
ω-3 PUFAs decrease the production of pro-inflammatory as mentioned above. However, there are controversial results in primary or cell line macrophages in different states of activation, which lead to variations in the production of inflammatory mediators. Inflammatory macrophages from fish oil-fed mice had lowered the output of TNF-α and IL-1β compared to control mice. In contrast, resident macrophages from fish oil-fed mice had increased the production of cytokines. These findings support the proposition that dietary ω-3 PUFAs may exert opposite effects depending on the activation status of macrophages [94].
The anti-inflammatory effects of ω-3 PUFAs in NF-κB signaling activation involve several molecular mechanisms [131]. GPR120 (G protein-coupled receptor for long-chain unsaturated fatty acids), recently renamed free fatty acid receptor 4 (FFAR4), is an n-3 PUFA receptor and has anti-inflammatory roles [132,133] in macrophages. Activation of GPR120 by ω-3 PUFAs leads to the internalization of this receptor and its binding to β-arrestin 2. The complex formed by GPR120 and β-arrestin 2 sequesters TAB1 (TGFβ1 activated kinase 1), impairs NF-κB pathway, and inhibits subsequent inflammatory events [96].
Treatment with ω-3 PUFAs limits inflammasome and TLR activation in macrophages. DHA also suppresses NF-κB activation and COX-2 expression induced by TLR-2 agonist [121] and by the agonist for TLR3, 4, 5, 6, or 9 [53] in a cell line macrophage. DHA inhibits NLRP3 inflammasome and subsequent caspase-1 activation [44,96] and IL-1β secretion [96,122].
In summary, dietary ω-3 PUFAs have anti-inflammatory properties, in part, due to competitive inhibition of macrophage arachidonic acid (20:4n−6) metabolism [134]. ω-3 PUFAs improve macrophage phagocytic activity (or remains unchanged). however, they impair their capacity to produce cytokines and NO by alleviating NF-κB signaling, TLRs, and NLRP3 inflammasome activation (please see Table 2 and Figure 1).
Dietary fat was considered only as a source of energy until 1929 when George and Mildred Burr reported that dietary fatty acid is required to prevent a deficiency disease that occurred in rats fed a fat-free diet [135]. They concluded that some fatty acids were essential nutrients, such as linoleic acid, which prevented the disease. They named this as an essential fatty acid [136,137].
Arachidonic (AA, 20:4 ω-6) and linoleic (LA, 18:2 ω-6) acids are the primary fatty acids of the ω-6 family. The major sources of linoleic acid are vegetable oils, nuts, seeds, meats, and eggs, except for coconut, cocoa, and palm [138]. AA is found predominantly in grain-fed animals, dairy, and eggs [139]. Human breast milk has high levels of ω-6 PUFAs [140]. These fatty acids are essential during early human life development to maintain the structure and function of cell membranes, tissues, and organs [141,142].
Linoleic acid is a precursor of arachidonic acid and bioactive eicosanoids [139,143]. Dietary linoleic acid is associated with tissue arachidonic acid content. Therefore, eicosanoid formation and subsequently enhance the inflammatory state intensity related to acute and chronic diseases [138].
Macrophages can present inflammatory profiles when exposed to metabolic stimuli, such as modified lipoproteins in atherosclerosis and diabetes mellitus. The microenvironment lipid content controls the activation state of macrophages to a pro-inflammatory state [144]. Besides the important role of lipid tissue in modifying macrophage profile, macrophages also accumulate in adipose tissue with increasing body weight and contribute to inflammation state in obesity [145].
Metabolic changes in the environment may stimulate non-classical inflammatory macrophages due to different forms of polarization and activation [126]. Once present in adipose tissues, macrophages exhibit levels of heterogeneity display in their activities and functions, reflecting the local metabolic and immune microenvironment [144,146–148].
Resident macrophages from rats fed with linoleic acid abundant diet have decreased the production of IL-1β, IL-6, and VEGF. However, in LPS-stimulated macrophages, linoleic acid promotes an increase of IL-1β release and reduces IL-10 production [97]. Despite LA ingestion not having modified the TNF-α, PGE2 and LTB4 production by peritoneal macrophages [98], the addition of gamma-linolenic acid (GLA) by gastroduodenal feeding catheter decreased PGE2 and LTB4 production in alveolar macrophages after LPS stimulation [90].
The mouse infections can be accompanied by an increase of ω-6 level in plasma, spleen, and liver [149], contributing to an inflammatory state. Mice treated with ω-6 PUFAs presented less control in the dissemination of Mycobacterium tuberculosis [95], but after AA treatment in vitro, the infection of J744 macrophages by M. tuberculosis decreased [95,150].
In the context of infections caused by pathogens in vitro (Rhodococcus equi and Pseudomonas aeruginosa), ω-6 PUFA family down-regulated inflammation process, decreasing IL-6, TNF-α, and IL-1β levels, and CD86 expression [112]. In the same way, treatment with ω-6 PUFA family (without infection) also decreased IL-6, TNF-α levels, and NADPH oxidase activity [113].
Treatment of LPS-stimulated RAW 264.7 macrophages with eicosadienoic acid (EDA) leads to an increase of TNF-α and PGE2 production and decreased NO synthesis. Macrophages rapidly incorporate EDA and increase the composition of AA. The percentages of both EDA and AA increase in cellular phospholipids in a dose-dependent manner. Modulation of PGE2 and NO production involves a change in the expression of COX-2 and iNOS [151]. Increased production of prostaglandins occurs when murine macrophages are treated with dihomo-γ-linolenic acid (DGLA) [152]. Besides LA, gamma-linolenic acid (GLA) treatment also attenuated the inflammatory state after LPS stimulation in RAW 264.7 macrophages, GLA decreased nuclear factor-κB (NF-κB), activator protein-1 (AP-1) and iNOS expression [153].
In addition to cytokine and NO production, ω-6 PUFAs alter respiratory burst and production of ROS. LA and AA treatment in vitro increased ROS [154] and NO production by RAW 264.7 macrophages [103]. However, LA and AA treatment did not affect respiratory burst in LPS-stimulated macrophages [103]. LA and AA increased (low concentrations) and decreased (high concentrations) NO production by murine cell line J774 [41]. On the other hand, supplementation with LA for ten days did not change the release of the superoxide anion, hydrogen peroxide, and nitrite by peritoneal macrophages [97].
Only a few studies reported LA induced changes in the production of cytokines. LA ingestion for 8 weeks did not affect the production of IL-6, TNF-α, IFN-γ, and IL-10 by peripheral blood mononuclear cells (PBMCs) even after fish oil ingestion [155]. AA increases the phagocytosis capacity of PBMCs isolated from healthy donors and cultivated during 7 days with 10% autologous serum [156]. LA treatment of RAW 264.7 macrophages [102] and mouse monocyte/macrophage line P388D1 [101] in vitro increased phagocytosis capacity.
Excessive levels of ω-6 PUFAs or low levels of ω-3 PUFAs results in an unhealthy ω-6/ω-3 ratio of 20:1, instead of 1:1 that is optimal for humans [157]. LA is the major ω-6, which generates arachidonic acid (AA) and subsequently PGE2, and LTB4 leads to increased production of IL-6, TNF-α, IL-1β, and NF-κB expression [158].
ω-6 fatty acid family reduces and alters the inflammatory grade, particularly after pathogen exposure. They increased the phagocytosis rate, bacterial killing, and decreased NOS and ROS. These findings remain controversial because each treatment depends on the period of treatment and stimulation conditions (please see Table 3 and Figure 1).
Some ω-9 MUFAs are abundant in animal fats and vegetable oils. The most abundant ω-9 MUFA is oleic acid (OA, 9-octadecenoic acid, 18:1, ω-9), which is the main component of olive oil [158].
A high membrane phospholipid composition of ω-9 MUFAs modulates fluidity and micro-domain structures that affect phagocytosis and respiratory burst [113,159,160]. Macrophages treated with OA have decreased adhesion capacity, which may be related to alterations in membrane fluidity and production of adhesion molecules [33,161]. In line with this finding, Lokesh and Wrann (1984) [31] reported impairment of sheep red blood cell phagocytosis by macrophages enriched with OA. Treatment of primary or cell line macrophages with OA increases phagocytosis [33,162], and fungicidal activity [33,163].
ω-9 MUFAs (more specifically, OA) have anti-inflammatory properties by decreasing the production of some mediators—as cytokines. Inhibition of tumor necrosis factor-α (TNF-α) secretion in many cell types [164] occurs as a consequence of OA treatment. The effects of ω-9 MUFAs on cytokine production depend on the concentration, period of treatment, cell type (whether it is primary or cell line), and activation state of macrophages. In vitro treatment with OA does not lead to alteration or reduction of steady-state production of interleukin-1β (IL-1β) [56,165], TNF-α [46,59,161,165–167], interleukin-6 (IL-6) [59,161,165,167] monocyte chemoattractant protein-1 (MCP-1) [165,168], interleukin-10 (IL-10) [59,165], leukotriene B4 (LTB4), prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), prostaglandin F2α (PGF2α), thromboxane B2 (TBXB2), 12-hydroxyeicosatetraenoic acid (12-HETE) and 15-hydroxyeicosatetraenoic acid (15-HETE) [165]. On the other hand, in some conditions, OA promotes augment in the production of TNF-α [46,161] and IL-10 [59]. Ex vivo treatment with OA also leads to reduction or no alteration of steady-state production of TNF-α [165,169], IL-1β, IL-6 and MCP-1 [165], but increases the production of IL-10 [165].
High intake of ω-9 MUFAs correlates with a lower risk of cardiovascular disease [170] by decreasing the production of inflammatory mediators. This beneficial effect of ω-9 MUFAs still requires confirmation. Supplementation with ω-9 MUFAs does not lead to alteration of plasminogen activator inhibitor-1 (PAI-1) production [171], reduction of PDGF-A and PDGF-B (both platelet-derived growth factors), MCP-1 [168,172], TNF-α [97,173], vascular endothelial growth factor (VEGF), PGE2, LTB4 [94,97] and 15-HETE [97] or increase of IL-10 [59,97,172]. On the other hand, specific supplementation protocols result in decreased production of IL-1β [66,94,97,162,174], cytokine-induced neutrophil chemoattractant-2αβ (CINC-2αβ) [97], and IL-6 [97,174]. The effect of OA on IL-6, LTB4, TNF-α, PGE2, and PGD2 production remains controversial since supplementation with this fatty acid increases IL-6 and LTB4 [173] and reduces TNF-α [91,94,174] production. OA can also lead to both decrease [91,94] and increase [175] of PGD2 as well as increase of PGE2 [175], depending on the activation state of macrophages. Tappia [173] reported a time-dependent pattern of OA actions: after 4-week supplementation, mice macrophages present decreased production of IL-1β without alteration in PGE2, whereas after 8-week supplementation there is a decrease of PGE2 and an increase of IL-1β.
The effect of OA on NO production depends on its concentration, macrophage cell type, and stimulation state. Treatment of RAW 264.7 macrophages with OA leads to a reduction of iNOS expression [165], with decreased [176] or no change in NO release [177] in LPS-stimulated cells. However, in non-stimulated J774 cells, low doses of OA (up to 50 μM) have been reported to increase both iNOS expression and NO release [41,46]. In primary macrophages, Magdalon [97] reported no change in NO production. The action of OA appears to be time-dependent: mice supplemented with OA for a short period display increased NO production [99] whereas mice supplemented for an extended period display decreased NO production [91,174].
Production of inflammatory mediators is closely related to the macrophage phenotype. The most recognized phenotypes are M1 and M2: in general, the first is proinflammatory, and the last is anti-inflammatory. ω-9 MUFAs alter the production of some mediators by macrophages, but the effect of ω-9 MUFAs on macrophage populations remains unknown. Few studies have reported that OA treatment increases the expression of CD206 [166], macrophage galactose-type lectin-1 (Mgl1), and arginase-1 (Arg1) [165,166], which drive macrophages to M2 phenotype. These cells display an anti-inflammatory feature that is characterized by increased production of anti-inflammatory mediators and decreased production of proinflammatory mediators. The impairment of cytokine and NO production by OA may be related to inhibition of NF-κB activation through the suppression of the TLR4 signaling pathway [121,161]. Although ROS and cytokine production is associated with NF-κB activation, there is no direct relationship between the modulation of ROS and cytokine release by fatty acids in the same way [41,161]. The effect of OA on TLR4 is not apparent, since both increase [177] and decrease [169] of its expression, as well as the rise of TLR4 migration into lipid rafts [178], have been reported. Concerning the TLR4-activated signaling pathway, OA decreases IκBα phosphorylation [56], which impairs NF-κB translocation [169] and activation [56]. There are reports on increased [41,178] or unaltered [179] NF-κB activity after OA treatment. Augment of PPARγ activity due to decreased acetylation [59] could also be involved in the reduction of NF-κB activation caused by OA. Despite the impairment of NF-κB signaling pathway and the increase of PPARγ activity, OA increases the expression of CD36. This receptor binds modified lipoproteins, necessary for the foam cell formation [180,181], and inflammatory mediators such as chemokine (C-X-C motif) ligand 1 (CXCL1), C-C motif chemokine 22 (CCL22) [166], and COX2 [182]. OA also reduces NLRP3 activation, possibly by impairing mechanisms upstream inflammasome assemblies, such as decreasing caspase-1 activation and pro-IL-1β cleavage [56]. The action of OA is specific for NLRP3, since other inflammasomes (NLRC4, NLRP7, and AIM2) are not affected by this fatty acid [56].
OA does not alter ROS production by both primary and cell line macrophages [99,179]. However, in a short-period treatment, this fatty acid increases superoxide production [161]. The augment of superoxide production may be due to the activation of protein kinase C, leading to an increase of NADPH oxidase activity [161,183]. In the presence of LPS, superoxide may be released to extracellular medium or degraded by antioxidant enzymes. One of these latter enzymes is superoxide dismutase (SOD) that converts superoxide anion to H2O2. This molecule is a more stable oxygen species associated with a potent microbicidal activity [161]. Treatment with OA increases H2O2 production in a dose-dependent manner, and this effect increases in the presence of LPS [161]. The increased H2O2 in extracellular medium occurs concomitantly with a decrease of intracellular superoxide anion. This fact supports the proposition that, in the presence of LPS or after a long period of OA treatment, superoxide anion produced inside the cell is converted by SOD to H2O2 and released into extracellular medium [161]. In line with these results, diets rich in ω-9 MUFAs increase SOD and glutathione peroxidase activities [184,185]. The effect of OA on ROS production in J774 macrophages may be related to the increased fungicidal activity. Diets enriched with OA may be beneficial to patients with diseases that require pathogen elimination [161]. However, increased ROS production due to dietary supplementation with OA is controversial. There are reports on both decreased H2O2 production [174] and no alteration of ROS release (both superoxide anion and H2O2) [31,97,99] due to the effect of OA.
OA alters macrophage percentage in different tissues, which contributes to modulate local immune response [186,187]. C3H/Hen mice supplemented with a high oleate sunflower oil (ω-9-rich diet) and challenged with Listeria monocytogenes present an increased percentage of peritoneal macrophages [186]. In mesenteric adipose tissue (MAT) of OA-supplemented mice, Camell [166] reported an increase of F4/80+ cells (macrophages). However, Shirakawa [171] did not observe the alteration of the total number of macrophages in epididymal adipose tissue (EAT). Therefore, the effect of OA seems to depend on the adipose tissue depot. None of these studies reported alteration in the percentage of CD11c+ macrophages (M1 macrophages) [166,171]. Also, Cardoso [188] reported that topic treatment with OA decreases the number of macrophages infiltrated into skin wounds, which leads to improvement of the wound healing process.
ω-9 MUFA actions vary according to treatment or supplementation (whether OA or olive oil). The dose used in the procedure, exposure period, cell type (whether it is primary or cell line macrophages), and activation state of macrophages, which explains the great diversity of findings reported in the literature (described in Table 4 and Figure 1).
OMEGA-7The primary fatty acid of this class is the monounsaturated palmitoleic acid (9-hexadecenoic acid) (16:1, ω-7). They are in ester form in triacylglycerol, phospholipids, and other glycerolipids of plants [189]. Macadamia seed and sea buckthorn oil [190–192] contain palmitoleic acid in the ester form. Palmitoleic acid is available from dietary sources, but it is also endogenously produced by adipocytes [193]. This fatty acid increases the fluidity of the cell membrane, inhibits the expression of oncogenes, and reduces inflammation associated with diabetes and the risk of heart diseases [194–197]. However, there are only a few studies regarding the effects of palmitoleic acid on the inflammatory response and, more specifically, on macrophage function.
The phagocytic activity of macrophages is closely associated with the structure of the cell membrane and to the fluidity determined by its lipid composition. The increase of macrophage phagocytosis activity depends on the degree of fatty acid unsaturation; the more unsaturated, the higher the phagocytosis activity. It could be explained by a decrease in the viscosity of the cell membrane, allowing rapid movements and diffusibility. Schroit & Gallily [163] reported that palmitoleic acid increases peritoneal macrophage phagocytic activity. However, the effects of linoleic acid (18:2, ω-6), α-linolenic acid (18:3, ω-3), and arachidonic acid (20:4, ω-6) are more pronounced.
Guo [193] reported that C57BL/6J mice fed a low-fat diet (LFD) or a high-fat diet (HFD) and supplemented with palmitoleic acid have a lower inflammatory response with a decrease in the number of macrophages/Kupffer cells in the liver [193]. Since macrophages/Kupffer cells play a critical role in the inflammatory response of the liver [198], suppression of their function may contribute to the anti-inflammatory effects of palmitoleic acid. This fatty acid has anti-inflammatory actions on macrophages. Guo [193] and Liu et al. [176] investigated the effect of palmitoleic acid at 50 or 200 μM on RAW 264.7 macrophages. The authors reported a decreased expression of proinflammatory cytokines such as TNF-α [176,193] and IL-6 [193]. There was also a reduction in the phosphorylation of NF-κβ at serine 468 of p65 subunit [193]. In line with these studies, treatment of both primary and cell line macrophages with palmitoleic acid reduces iNOS expression and NO release [42,199] and productions of TNF-α [42,199], IL-6 [199] and MCP-1 [42]. Treatment of macrophages with palmitoleic acid also increased the expression of M2 genes as Mrc1, Tgfb1, Il-10, and Mgl2 [199]. These anti-inflammatory effects are mediated by reduction of IκBα degradation and NF-κB p65 nuclear translocation, both dependent on AMPKα [199].
Interest in the effects of ω-7 MUFAs has grown since they seem to invoke anti-inflammatory effects and, thus, might be helpful in the treatment of associated inflammatory diseases. However, they are still under-investigated (see Table 5 and Figure 1 for a summary of effects), and more studies are necessary to unravel their actions on leukocytes, and more specifically, on macrophages.
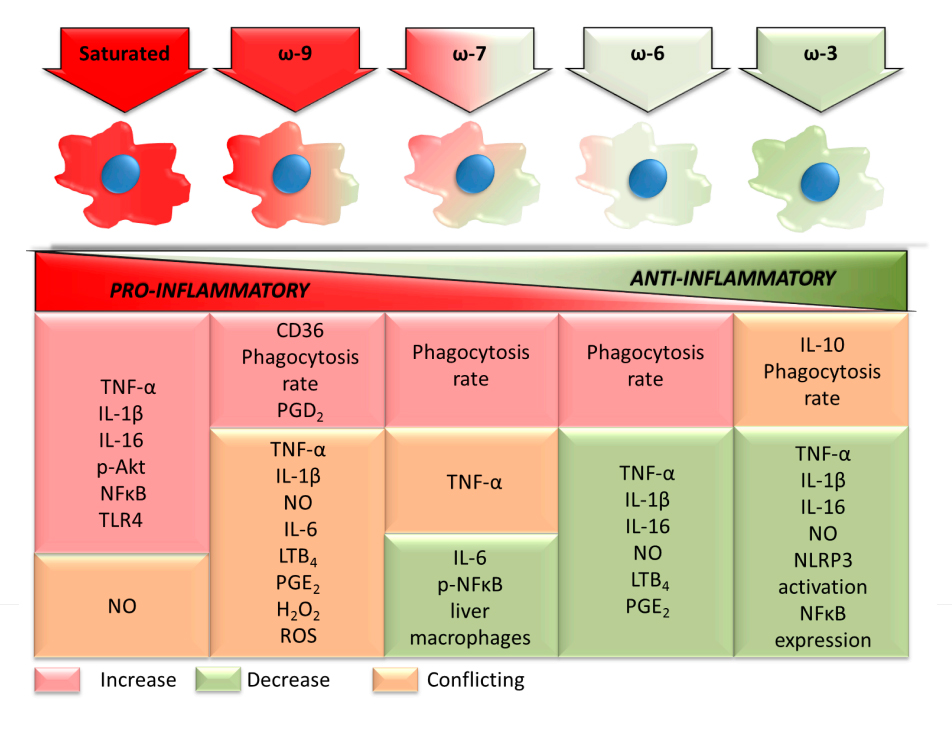 Figure 1. Pro- and anti-inflammatory effects of the main dietary fatty acids on macrophage functions. TNF: tumor necrosis factor; IL: interleukin; p: phospho; Akt: protein kinase B, PKB; NF-κB: nuclear factor kappa B; TLR: toll-like receptor; NO: nitric oxide; CD: cluster of differentiation; PG: prostaglandin; LT: leukotriene; H2O2: hydrogen peroxide; ROS: reactive oxygen species; NLRP3: NACHT, LRR and PYD domains-containing protein 3.
Figure 1. Pro- and anti-inflammatory effects of the main dietary fatty acids on macrophage functions. TNF: tumor necrosis factor; IL: interleukin; p: phospho; Akt: protein kinase B, PKB; NF-κB: nuclear factor kappa B; TLR: toll-like receptor; NO: nitric oxide; CD: cluster of differentiation; PG: prostaglandin; LT: leukotriene; H2O2: hydrogen peroxide; ROS: reactive oxygen species; NLRP3: NACHT, LRR and PYD domains-containing protein 3.
A summary of the effects of the fatty acid classes on the macrophage inflammatory state herein reported is in Figure 1. The changes in cytokine production, prostaglandin production, phagocytosis capacity, NO production, NF-κB activation, and NLRP3 activation induced by each fatty acid class are in Figure 1. The changes are indicated by increase, decrease, or conflicting. This latter term means that the literature remains undecided about the findings reported. The fatty acid classes were ordered, considering the inflammatory properties. From the pro- to anti-inflammatory fatty acids saturated, ω-9 monounsaturated, ω-7 monounsaturated, ω-6 polyunsaturated, and ω-3 polyunsaturated. The opposite sequence applies to the anti-inflammatory properties of the fatty acid classes. Some discrepancies may exist between the macrophage inflammatory state and the systemic inflammatory properties of the fatty acid classes. The reported pro-inflammatory effects of the saturated fatty acids and the anti-inflammatory properties of the ω-3 polyunsaturated fatty acids are widely reported in a variety of experimental models and disease conditions.
MRD and ARC performed the bibliographical search, and design and generated all the tables. MRD, ARC, PN, and RC wrote this review. GM designed and generated Figure 1. All authors have seen and approved the final version of the manuscript.
The authors declare that they have no conflict of interest.
The authors’ findings reported in this review were obtained using financial support from CAPES, CNPq, FAPESP and Diabetes Australia Research Trust (DART) (grant number Y16G-MAMC).
The review was completed while PN was Academic Program Study (APS) leave from the School of Pharmacy and Biomedical Sciences, Curtin University, their support is gratefully acknowledged.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
87.
88.
89.
90.
91.
92.
93.
94.
95.
96.
97.
98.
99.
100.
101.
102.
103.
104.
105.
106.
107.
108.
109.
110.
111.
112.
113.
114.
115.
116.
117.
118.
119.
120.
121.
122.
123.
124.
125.
126.
127.
128.
129.
130.
131.
132.
133.
134.
135.
136.
137.
138.
139.
140.
141.
142.
143.
144.
145.
146.
147.
148.
149.
150.
151.
152.
153.
154.
155.
156.
157.
158.
159.
160.
161.
162.
163.
164.
165.
166.
167.
168.
169.
170.
171.
172.
173.
174.
175.
176.
177.
178.
179.
180.
181.
182.
183.
184.
185.
186.
187.
188.
189.
190.
191.
192.
193.
194.
195.
196.
197.
198.
199.
Davanso MR, Crisma AR, Murata G, Newsholme P, Curi R. Impact of Dietary Fatty Acids on Macrophage Lipid Metabolism, Signaling and Function. Immunometabolism. 2020;2(1):e200008. https://doi.org/10.20900/immunometab20200008

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions