Location: Home >> Detail
TOTAL VIEWS
Immunometabolism. 2020;2(1):e200007. https://doi.org/10.20900/immunometab20200007
Department of Immunology and Pathology, Monash University, Melbourne, VIC 3004, Australia
* Correspondence: Nicola Harris.
That metabolic phenotype can dictate the function of macrophages has been widely demonstrated in vitro, however in vivo relevance of these findings has been lacking. Sverdberg et al., observe that the in vivo microenvironment shapes the ability of macrophages to utilize glucose and thus affects their responsiveness to stimuli.
Macrophages are present in virtually all mammalian tissues, and have a plethora of functions including monitoring of local environment, maintaining homeostasis, wound healing, embryonic development and defence against infection [1]. During embryogenesis, macrophage precursors originate either from the yolk sack or the foetal liver to form resident macrophage populations in the tissues. Cellular ontogeny can impact on macrophage function as can factors present within the local tissue environment [2,3]. As a result, macrophages display phenotypic heterogeneity and are associated with a range of tissue-specific functions [2]. Cellular metabolism can also shape macrophage function. Exposure to a type 1 or pro-inflammatory signal promotes a switch from mitochondrial oxidative phosphorylation (OXPHOS) to glycolysis [4,5]. Glycolysis allows for the rapid production of ATP to meet the high energy demand of M1/inflammatory macrophages, which is required during the phagocytosis of pathogens. Conversely, macrophages exposed to anti-inflammatory signals, or the type 2 cytokine IL-4, display enhanced OXPHOS [6,7], thought to allow sustained energy production. However, these studies largely rely on in vitro culture of bone marrow derived macrophages [6,8–11], and in vivo validation of the link between cellular metabolism and function has been lacking.
The lung harbors several macrophage populations including interstitial (or tissue) macrophages (IMs) and alveolar macrophages (AMs), present in distinct anatomical sites (parenchyma versus airways). AMs are reported to be involved in the monitoring of the airways and regulation of surfactant homeostasis [12], whilst the function and phenotype of IMs remain poorly defined [12]. In a recent article, published in Nature Immunology, Svedberg et al. [13], found that the alveolar space and lung parenchyma represent unique environmental niches that dictate the function of AMs and IMs, particularly their ability to respond to IL-4 (Figure 1) [13].
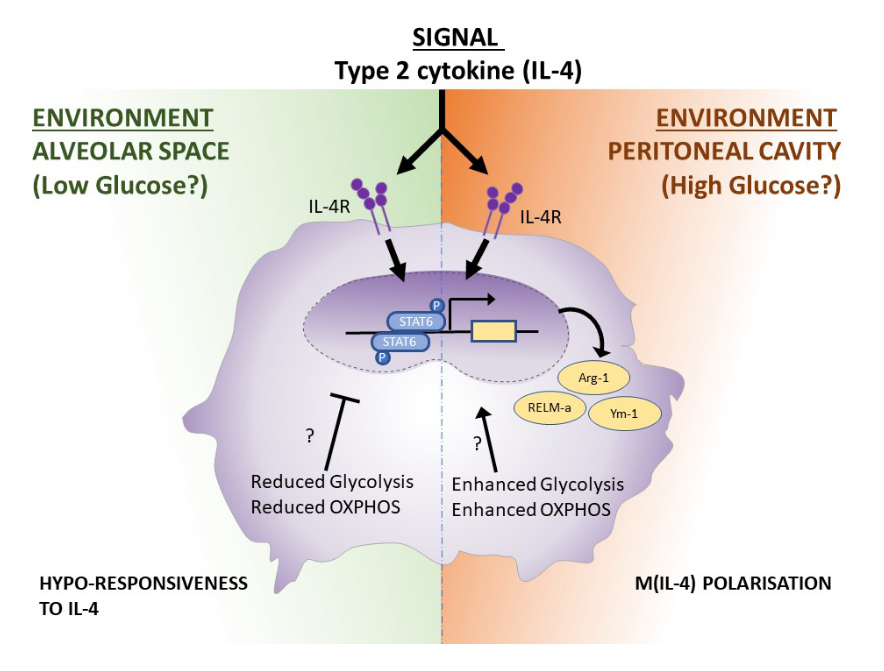 Figure 1. Tissue-specific metabolic adaptations of macrophages shape their responsiveness to IL-4. Alveolar macrophages and peritoneal macrophages differ in their metabolic programming due to the specificities of their niche. Because of this metabolic poise, upon IL-4 stimulation while both macrophage (M(IL-4)) population can undergo STAT6 phosphorylation, the downstream M(IL-4) hallmark genes Arginase-1 (Arg-1), RELM-a and Ym-1 are expressed only in peritoneal macrophages, while alveolar macrophages fail to upregulate their expression. The authors speculate that the glucose level in each niche might be the main driver of this phenotype. How the tissue specific metabolic reprogramming can impact positively or negatively on M(IL-4) gene expression is still an open question.
Figure 1. Tissue-specific metabolic adaptations of macrophages shape their responsiveness to IL-4. Alveolar macrophages and peritoneal macrophages differ in their metabolic programming due to the specificities of their niche. Because of this metabolic poise, upon IL-4 stimulation while both macrophage (M(IL-4)) population can undergo STAT6 phosphorylation, the downstream M(IL-4) hallmark genes Arginase-1 (Arg-1), RELM-a and Ym-1 are expressed only in peritoneal macrophages, while alveolar macrophages fail to upregulate their expression. The authors speculate that the glucose level in each niche might be the main driver of this phenotype. How the tissue specific metabolic reprogramming can impact positively or negatively on M(IL-4) gene expression is still an open question.
In type 2 cellular immune responses, such as allergy or helminth infection, IL-4 production results in the polarization of macrophages resulting in a distinct phenotype M(IL-4) characterised by the expression of arginase-1 as well as factors involved in tissue repair, and anti-inflammatory mediators [14]. Using markers recently described [2,15,16], and highly specific, gating strategy to differentiate IMs and AMs, Svedberg et al. [13], observed that AMs were less responsive than IMs in vivo, following systemic or local administration of IL-4. AM hypo-responsiveness to IL-4 was confirmed in mice infected with the rodent helminth Nippostrongylus brasiliensis, which elicits the potent production of IL-13 and IL-4 in the lung resulting in the activation of M(IL-4) that facilitate tissue repair and provide protection against reinfection [17,18]. Svedberg et al. [13], report that AMs, contrary to IMs, fail to expand or express typical markers of M(IL-4) following infection. The differential responsiveness of AM and IM to available IL-4 could result from the distinct ontogeny or environmental niche of these cells. To address this issue, Svedberg et al. [13] purified AMs from the lungs of naive mice and cultured them in vitro. Cultured AMs altered their gene expression profile and regained responsiveness to IL-4. This key experiment provides proof that the alveolar space constitutes a unique environment responsible for dictating the in vivo responsiveness of these cells. This finding was further corroborated by an experiment in which peritoneal macrophages (PECs) were delivered by intranasal instillation into naïve mice, following which they adopted a phenotype similar to that of AMs, and failed to respond to IL-4.
By comparing the transcriptomic profile in response to IL-4 of AMs, IMs and PECs, Svedberg et al. [13], identified that metabolic reprogramming occurs in PECs but that few metabolic pathways were altered in response to IL-4 in IMs and AMs. In depth comparison of PECs and AMs revealed a significantly reduced expression of genes involved in glycolysis and TCA cycle by AMs. To confirm that the metabolic profile of PECs and AM were distinct, the authors used flux analysis. Both glycolysis and OXPHOS were measurably decreased in AMs compared to PECs, with AMs exhibiting lower glucose uptake in vitro.
As reduced OXPHOS could explain the hypo-responsiveness of AMs to IL-4 the authors performed experiments in which in vitro cultured AMs were treated with etomoxir to block mitochondrial respiration. Etomoxir, or 2[6(4-chlorophenoxy) hexyl] oxirane-2-carboxylate, is an irreversible inhibitor of the transporter carnitine palmitoyltransferase-1 on the outer face of the inner mitochondrial membrane, which in turn prevents fatty acid β-oxidation. Addition of etomoxir to cultures only partially recapitulated the in vivo phenotype of AMs indicating that altered OXPHOS did not underlie the reduced responsiveness of AMs to IL-4. Interestingly, these data are in contrast to established results in bone-marrow derived macrophages [19,20] highlighting the need for studying immunometabolism in different macrophage populations. There is, however, some controversy as to whether etomoxir-induced loss of M(IL-4) polarisation is OXPHOS dependent with a recent study reporting that high concentrations of etomoxir additionally perturb intracellular CoA homeostasis [21]. OXPHOS metabolism in AMs has previously been reported to be enhanced as compared to IMs [22,23], due to the degradation of the surfactant molecule (SP-A/SP-D) in the airways which are enriched in lipids. Enhanced OXPHOS in AMs, as compared to IMs, was proposed to explain why these cells are able to sustain their energy production in a glucose-deprived environment [22]. In the light of those studies, it would be interesting to further address the importance of OXPHOS in the hypo-responsiveness to IL-4 of AMs using other inhibitors.
Svedberg et al. [13], next determined whether the lower expression of genes involved in glycolysis in AMs versus PECs, contributed to their altered phenotype. To investigate this, mice were injected intraperitoneally with a glucose analogue, 2-NBDG and its uptake by different macrophage populations was assessed. PECs transferred to the alveolar space recapitulated the phenotype obtained with resident host AMs by displaying low glucose uptake. Whilst this experiment suggests that low glucose uptake, and the resultant reduction in cellular glycolysis, contributes to the phenotype of AMs, it was not clear whether the intraperitoneally-injected glucose become readily availability within the lung alveolar airspace. In a second experimental system Svedberg et al. [13], treated AMs in vitro with 2DG to block glycolysis. Whilst cultured AMs regain their responsiveness to IL-4, AMs treated with 2DG failed to do so, corroborating the hypothesis that reduced glycolysis contributes to the unresponsiveness of AMs to IL-4 [9,24]. 2DG can also impact OXPHOS [25], however since interference of the OXPHOS pathway with etomoxir did not impact on the phenotype of AM it is more likely that the ability of 2DG to block glycolysis is responsible for its effect. While the authors did not identify the specific component of the niche that drives the hypo-responsiveness of AMs to IL-4, a possibility is that the low glucose availability in the airways [26] predisposes these cells to rely less heavily on glycolysis. Indeed, Davies et al, 2018 [27] suggest that the metabolite ‘richness’ of a niche could contribute to the responsiveness of macrophages. In this manuscript, the peritoneal cavity forms a niche rich in amino-acids, particularly glutamate, as compared to the serum. The authors of this study further show that the PECs exploit this glutamate-rich environment in a poised response against microbial stimuli.
One caveat of studies addressing the importance of niche factors in driving immune responsiveness is the difficulties associated with comparing macrophages derived from different tissue compartments. For instance, the authors chose to compare AMs to PECs and noted reduced OXPHOS in AMs, however AMs have previously been reported to display enhanced OXPHOS as compared to IMs [22]. Of note, the choice of IMs, rather than AMs, as the reference population would probably alter the conclusions drawn in the manuscript. Until recently, most studies investigating macrophage immunometabolism had utilized bone-marrow derived macrophages. This system allows controlled investigation of the impact of specific genes and conditions on cellular metabolism. By contrast, determining the most relevant reference population for in vivo studies is fraught with biases that are not easily addressed. One, albeit far from perfect, solution could be to compare unstimulated AMs to IL-4 stimulated AMs rather than IL-4 stimulated AMs to IL-4 stimulated PECs. Alternatively, multiple reference populations could be included, in this case IMs, PECs and bone-marrow derived macrophages.
Nevertheless, further work determining M(IL-4) polarisation in response to different doses of glucose, as well as other metabolites, will be crucial to validate these observations and to further understand the implications of metabolite availability in shaping tissue macrophage responsiveness in vivo. One of the questions emerging from the work of Svedberg et al. [13], 2019 pertains to the functional relevance of IL-4-mediated airway hypo-responsiveness. The airways lie at the interface between the host and the environment, and as such, represent a first line of defence against microbial and pollutant exposure. Giving this crucial role, one might speculate that AMs are poised to respond to type1 stimuli at the cost of not being able to mount a potent type 2 response. In this context, it would be interesting to determine whether AMs are hyper/hypo-responsive to IFNg or LPS. An alternative interpretation might be that the hypo-responsiveness to IL-4 has evolved to limit the pathologies associated with uncontrolled or persistent type 2 immune responses, such as asthma. In line with this idea, it is interesting to note that whilst AMs do not respond to IL-4, resting AMs express many genes usually considered as being part of the type 2 profile.
The importance of work addressing the role of the niche factors in macrophage polarisation is highlighted by the increasing interest in targeting macrophages to promote wound repair, improve infection outcomes or prevent fibrosis [12,28]. As we develop a greater understanding of macrophage metabolism and how it shapes the response of these cells, we may also be able to harness immunometabolism for therapeutic purposes [19,29,30]. Evidence stemming from cancer therapeutics indeed show that alteration of tissue metabolism can influence pathophysiological and immunological responses. One of these examples is treatment with Metformin, which modulates FAO, that has been shown to improve clinical outcomes in both cancer and in pre-clinical models of tuberculosis [31]. In terms of respiratory disease, elevated glucose in the airways has been associated with pathological conditions associated with uncontrolled type 2 responses including asthma and Chronic obstructive pulmonary disease), and it is tempting to speculate that treatments interfering with glucose uptake or metabolism could restore the IL-4 hypo-responsiveness of AMs and potentially alleviate disease symptoms. Such treatments (e.g., 2-DG, Ritonavir which affects glucose transporter-1 or FXII that targets lactate dehydrogenase) have already been used pre-clinically in cancer models, and are ripe for application in other disease contexts. The challenge for the future will be in leveraging the knowledge of how macrophages metabolically adapt to unique tissue niches, and how we can exploit this knowledge to develop tissue-specific therapeutic strategies.
The authors declare that they have no conflicts of interest.
N.L.H. is supported by a National Health and Medical Research Council (NHMRC) of Australia SRF-B fellowship.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
Bouchery T, Coakley G, Harris N. Tissue location drives the metabolic re-profiling of macrophages. Immunometabolism. 2020;2(1):e200007. https://doi.org/10.20900/immunometab20200007

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions